
Echinacea Purpurea Extract
Echinacea Purpurea Extract
Echinacea Purpurea Extract Latin name: Echinacea purpurea (Linn.) Moench Supported Purity: Polyphenol 4% by UV /chicoric acid 1%~10% by HPLC/ Concentrated extract by TLC Appearance: Yellow-green fine powder Material Original: QingLing Mountain, China
Echinacea Purpurea Extract Specification Supplied by MIGU:
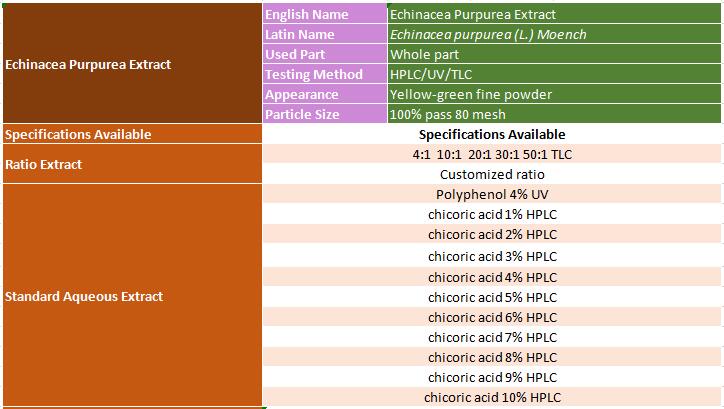
Echinacea Purpurea Extract Specification
Brief Introduction:
Echinacea Purpurea ( latin name: Echinacea purpurea (L.) Moench) is well-known and famous herbs plant used in medicinal in world. It belongs the Asteraceae (Compositae) family. This plant is widely planted for medicinal mainly used for chemical prevention and chemotherapy of infectious diseases of upper and lower respiratory system. In traditionally history, this plant commonly used in the treatment of snake bite, skin disorders, toothache, seizure, bowel pain, chronic arthritis and cancer. Nowadays, after the clinical study the purple coneflower can stimulate the immune system,upper respiratory tract infections including colds. Although the isolation and structural elucidation of its main compounds have been noticed by investigators, there is no affirmation about its mechanism of action. Alkamides, caffeic acid derivatives, and polysaccharides have been considered important constituents of the plant. A number of studies revealed that alkamides are involved in the immunomodulatory properties of Echinacea extracts in vitro and in vivo Additionally, caffeic acid is found in some species of Echinacea and could be applied toward authentication and quality control of the plant extracts. The polysaccharides play an important role in the anti-inflammatory effect of Echinacea preparations.Taxonomic, chemical, pharmacological, and clinical characteristics of some species of the Echinacea genus including E. angustifolia, E. pallida, and E. purpurea were reviewed in previous papers.Medicinal properties of the plant were also considered in a review paper, which suggested that more research is required for more definitive medicinal recommendations.

echinacea purpurea extract
Pharmacological Activity and Biological Activity for Echinacea Purpurea Extract :
Immunomodulatory effects
The function of immunomodulatory effects for Echinacea Purpurea Extract mainly caused by three mechanisms: Phagocytosis activation, fibroblast stimulation, and the enhancement of respiratory activity that results in augmentation of leukocyte mobility.There are numerous in vivo studies on the immunomodulatory and anti-inflammatory effects of E. purpurea that suggest that innate immunity is enhanced by administration of the plant and that the immune system is strengthened against pathogenic infections through activation of the neutrophils, macrophages, polymorphonuclear leukocytes (PMN), and natural killer (NK) cells.For this reason, it can be suitable for prevention against and treatment of various infectious diseases such as infections of the upper and lower respiratory systems, wound infections, and chronic pelvic infections.The complex chemical composition of the roots and herbs of Echinacea involves alkamides ketoalkenes, caffeic acid derivatives, polysaccharides, and glycoproteins, which are believed to be responsible for noted immunostimulatory and anti-inflammatory activities.Moreover, alkamides are demonstrated to be effective on cannabinoid receptor type 2 (CB2), and this is considered as a possible mechanism of their immunomodulatory properties.Their possible molecular mechanism could be the increase of cyclic adenosine monophosphate (cAMP), p38 mitogen-activated protein kinases (p38/MAPK), and c-Jun N-terminal kinases (JNK) signaling, as well as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), activating transcription factor 2/cAMP responsive element binding protein 1 (ATF-2/CREB-1) in primary human monocytes and macrophages.
Anti-inflammatory effects
The Echinacea preparation, interestingly, has reversed the inflammation caused by some bacteria in a culture of epithelial cells by reducing cytokines.Carrageenan-induced paw edema in experimental animals is a test for the evaluation of anti-inflammatory effects of plants extracts.Dried root powder of the plant, administered to the mice (30-100 mg/kg), inhibited carrageenan-induced paw edema similar to indomethacin. This effect may be attributed to the inhibition of COX-1 and to a lesser extent COX-2 by alkamides. Also, it is reported that the ethanol extracts of both aerial parts and roots of the plant have inhibited fibroblast-induced collagen concentration. The effects of Echinacea and some of its compounds on NF-κB expression by Jurkat cells (a human T-cell line) were assessed in the presence and absence of stimulation by LPS and phorbol 12-myristate 13-acetate (PMA). NF-κB is a nuclear transcription factor stimulating the expression of some genes such as key factors of inflammation, e.g., TNF-α, IL-1, chemokines, adhesion molecules, and COX-2. In the absence of stimulants, all root extracts and compounds did not show a significant effect on NF-κB expression. In fact, LPS decreased NF-κB expression, while this effect was significantly reversed in the presence of root extracts, cichoric acid, and the alkamides fractions. Cichoric acid and a 2,4-diene alkamide are demonstrated to significantly increase NF-κB levels, while a 2-ene alkamide caused a significant inhibition, indicating considerable diversity in the bioactivity of this plant.
Psychoactive activity
The anxiolytic activity of Echinacea drugs was determined in experimental animals with lower doses than those used in traditional indications.Alkamides of Echinacea have been reported to have cannabinomimetic properties on both cannabinoid CB1 and CB2 receptors, which may be attributed to their structural similarity to the endogenous cannabinoid receptor ligand anandamide. Anandamide’s effects are mediated in the brain and periphery by CB1 and CB2 receptors. The activation of the former with endogenous ligands plays a considerable role in controlling anxiety and the latter is mainly involved in immune system activities. Plants rich in alkamides induce paresthesia and were applied by Native Americans traditionally and also by physicians in the early 20th century as a sialogogue, an antitussive, and a remedy for toothache. The slight differences in the structures of the alkamides revealed significant differences in CB receptor activity, for instance the replacement of isobutylamide moiety of the dodeca-2E,4E,8Z,10Z/E-tetraenoic acid isobutylamide for 2-methylbutylamide moiety turned the G-protein stimulation property from inverse agonist to partial agonist. However, the extracts of E. purpurea were not active in the antiacetylcholinesterase (antiAChE) assay.The plant tincture increased the stimulation effect of L-3,4-dihydroxyphenylalanine (L-DOPA) and showed antidepressant activity in the clofelin-induced depression test in white rats.

echinacea purpurea extract planting base
Antioxidant activity
In some studies, radical-scavenging activities of the plant extracts were tested. For this plant, antioxidant activity compared with the alkamides and cichoric acid that are the characteristic compounds of Echinacea. The free radical-scavenging activity of the extracts was related to their cichoric acid contents, while alkamides were inactive against free radicals.Although in some studies it was revealed that the extract of the plant did not show any antioxidant property, other investigations of the root extract of the plant revealed antioxidant activity,which may be attributed to the phenolic contents and cichoric acid in the plant. Cichoric acid shows an efficient radical-scavenging activity toward 2,2-diphenyl-1-picrylhydrazyl (DPPH), comparable to flavonoids and rosmarinic acid. Although alkamides have not exhibited antioxidant activity, they are able to increase the activity of cichoric acid through two mechanisms: First, surface activity that enables cichoric acid to have better access to inhibit lipid oxidation in the lipophilic droplets of emulsion, and second, regeneration of cichoric acid by donating allylic hydrogen to the one-electron oxidized cichoric acid.
Antibacterial and antifungal activity
The extract of E. purpurea considerably inhibited growth of Candida albicans and Saccharomyces cerevisiae, but no inhibition zone was observed for Aspergillus niger. The extract obtained by classical method showed higher antimicrobial activity in comparison with those obtained by ultrasound-assisted extraction. In another study, three pathogens Streptococcus pyogenes, Haemophilus influenzae, and Legionella pneumophila were sensitive to the preparation of the plant, although Acinetobacter baumannii, Bacillus cereus, Bacillus subtilis, Enterococcus faecalis (vancomycin-resistant), Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa were relatively resistant to the drug, while two fungi organisms, C. albicans and Trichoderma viride, were essentially resistant to the preparation.The hexane extract of the root inhibited both yeasts S. cerevisiae and C. albicans. Additionally, 1-Tridecene-3,5,7,9,11-pentayne, a polyacetylenic compound from root of the plant has showed light-mediated inhibition toward S. cerevisiae.
Antiviral activity
The aqueous extract of E. purpurea were active against acyclovir-susceptible and acyclovir-resistant strains of both herpes simplex virus 1 (HSV-1) and herpes simplex virus 2 (HSV-2) in vitro, whereas the hexane extract of the plant root and cichoric acid inhibited HSV-1. In addition, cichoric acid inhibited human immunodeficiency virus type 1 (HIV-1) integrase.Mouse embryonic fibroblasts incubating with the plant juice and alcoholic root extract were resistant to influenza A2, herpes, and vesicular stomatitis virus infection for 24 h. The standard preparation of the plant showed strong inhibition against the influenza viruses A/Victoria/75 (H3N2) and A/Puerto Rico/8/1934 (H1N1), avian strains A/Thailand/1(KAN-1)/2004 (H5N1) and A/FPV/Dutch/1927 (H7N7), and the pandemic novel swine-origin influenza A (S-OIV) (H1N1) in direct contact. Moreover, the results of hemagglutination (HA) assays indicated that the preparation inhibited HA activity and could therefore block entry of a virus into treated cells.The administration of a polysaccharide extract of the plant to infected mice with influenza A H1N1 (A/WSN/33) caused weight loss, but similar pulmonary viral titers were observed in comparison with untreated mice. Lower systemic and pulmonary keratinocyte chemoattract (KC) and interleukin 10 (IL-10) levels, and systemic levels of IFNγ were observed in treated mice, indicating that E. purpureacould modulate the clinical symptoms of influenza by altering cytokines.Based on the results of the two latter studies, it seems that different components of the plant have beneficial effects on influenza patients through different mechanisms.
Mutagenicity
The extract of E. purpurea flower did not exhibit mutagenicity against Salmonella typhimurium TA98 and TA100 with or without the S9 fraction. It also showed a dose-dependent inhibition against the mutagenicity of 2-aminoantheracene, therefore it could be a good antimutagenic agent. High concentrations of the plant (8 mg/ml) reduced sperm motility and sperm penetration of hamster oocytes attributed to sperm DNA denaturation, while these effects were not observed by administrating low concentrations.
Toxicology
Generally, animal studies of various preparations of Echinacea species have shown low toxicity. In a study of acute toxicity, LD50 value was calculated as 2500 mg/kg in an intraperitoneal injection of the polysaccharide fraction of the plant in female mice.In other studies, the LD50 values of oral and intravenous administration of the plant juice evaluated more than 30 g/kg and 10 g/kg in mice, and 15 g/kg and 5 g/kg in rats, respectively.
Clinical trials
The findings of clinical trials of the plant preparation are controversial. The results of a randomized blinded trial revealed no significant difference in the incidence and severity of colds and respiratory infection between Echinacea and placebo groups in 108 patients during 8 weeks of administration of the plant juice. Only a small decrease for the total lymphocyte counts was found, which may have occurred by chance rather than because of a true effect of the plant juice or placebo. On the other hand, the results of a study showed that preparations of E. purpurea had a beneficial effect on adults with cold symptoms in clinical trials, especially in comparison to placebo, if the treatment is begun early.
Adverse effects
In several clinical studies, the frequency of adverse effects in the Echinacea group and control group were similar and not statistically significant, while in some studies, rash and gastrointestinal symptoms were reported from the Echinacea group. Some adverse effects were also reported by the UK Committee on Safety of Medicines and the Medicines and Healthcare Products Regulatory Agency’s spontaneous reporting scheme (the “yellow card” scheme), commonly including abdominal pain, angioedema, dyspnea, nausea, pruritus, rash, erythema, and urticaria.
Contraindications
The Echinacea preparations are contraindicated in some patients including those with progressive systemic diseases such as tuberculosis, leukemia and leukemia-like diseases, collagen disorders, multiple sclerosis, and other autoimmune diseases.Some Echinacea products are also contraindicated in AIDS and HIV infections. These statements are based on the theory of immunumodulatory activity of Echinacea, although there is an opposing idea that these products are not harmful in patients with autoimmune diseases. There are no clinical data available supporting the mentioned views, but it is recommended to avoid these preparations in immune-related diseases.The preparations of Echinacea should be administered with caution concomitantly with immunosuppressant drugs.Effects of some preparations made from the root and herb of E. purpurea along with cichoric acid were tested on the human drug-metabolizing enzyme cytochrome P450 3A4 (CYP3A4). The results indicated that the preparations moderately inhibited the enzyme, while cichoric acid showed low inhibition activity.The findings of a study of healthy nonsmoking volunteers showed that the plant root remedy inhibited cytochrome P450 1A2 (CYP1A2) and intestinal CYP3A4 but not cytochrome P450 2C9 (CYP2C9) or cytochrome P450 2D6 (CYP2D6), whereas induced hepatic CYP3A4 activity. The selective effects of the plant on CYP3A4 activity may be explained by several mechanisms: The constituents of the plant, that are responsible for CYP3A4 inhibition are not systematically available; the constituents that are responsible for CYP3A4 induction may rapidly be absorbed, leading to a lack of intestinal CYP3A4 induction; hepatic CYP3A4 induction may occur by the metabolites of the plant, or the CYP3A4 induction may involve tissue activators that are influenced by constituents of the plant. Additionally, the plant inhibited metabolism of testosterone by CYP3A4 via nicotinamide adenine dinucleotide phosphate (NADPH)-dependent reaction.More Scientific research and development for echinacea purpurea extract, please check here
Other fungus extract like reishi, cordyceps, maitake, shiitake, chaga can be provided by MIGU.
Reference:















